Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare and life-threatening hematologic disease characterized by intravascular and extravascular hemolysis (IVH and EVH) mediated by complement activation through both the terminal and alternative pathways (TP and AP). The current treatment options for PNH are limited to addressing only a single complement pathway, resulting in either unresolved EVH with TP inhibitors or potentially exposing patients to the risk of life-threatening breakthrough hemolysis with AP inhibitors (Notaro et al. N Engl J Med 2022;387:160-6). KP104 is an innovative fusion protein comprised of a humanized anti-C5 mAb and the functional domain of complement regulator factor H to inhibit both TP and AP activation. We report here the interim results from a phase 2 study of KP104 in complement inhibitor-naïve PNH patients.
Methods
This open-label Phase 2 clinical trial (NCT05476887) aims to assess the efficacy, safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of KP104 in patients diagnosed with PNH who have not received prior complement inhibitors. The primary objectives of the study are to evaluate key clinical markers including lactate dehydrogenase (LDH) and hemoglobin (Hgb) levels, transfusion requirements, and FACIT-fatigue scores. The first part of the study consists of three cohorts, with an enrollment of 6 patients per cohort. The study includes an initial treatment period of 12 or 13 weeks of KP104 dosing regimen, followed by a 9-month extension treatment period. The specific dosing regimens for each cohort are as follows:
Cohort 1: 1,200 mg IV (Day 1) + 720 mg SC QW (starting Day 8)
Cohort 2: 2,400 mg IV (Day 1) + 1,920 mg SC Q2W (starting Day 8)
Cohort 3: 3,600 mg IV (Day 1) + 2,880 mg SC Q2W (starting Day 8)
Results
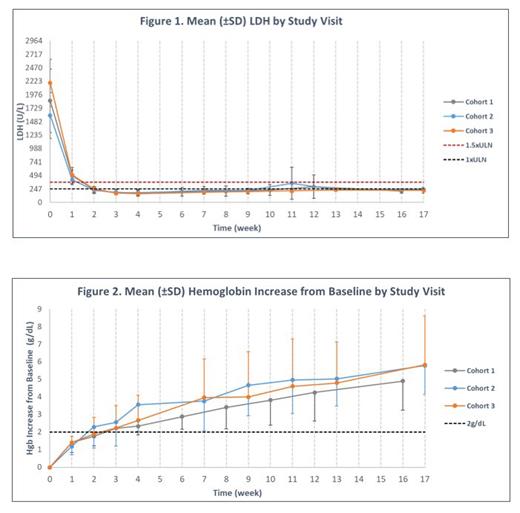
Interim results include data from 15 patients who completed 16/17 weeks of treatment (6 patients each from Cohort 1 and 2, and 3 from Cohort 3), as of the data cutoff on July 12 th, 2023. Among these patients (4 males and 11 females, mean (±SD) age 33(±12) years, a median of 3 transfusions were required 12 months prior to the study and 9 patients were previously diagnosed with aplastic anemia. Baseline mean (SD) Hgb and LDH levels were 7.0 (±1.5) g/dL and 1,824 (±512) U/L, respectively.
By week 16/17, all 15 patients demonstrated positive clinical improvements. Mean (SD) Hgb levels increased by 4.9 (±1.7) g/dL, 5.8 (±1.6) g/dL, and 5.8 (±2.8) g/dL over baseline, and mean (SD) LDH levels reduced by 88.0 (±3.67) %, 83.5 (±7.45) %, and 89.5 (±4.05) % over baseline for Cohort 1, 2 and 3, respectively. Additionally, 9/15 (60%) patients achieved hemoglobin levels ≥12 g/dL (3/6, 5/6 and 1/3 for Cohort 1, 2 and 3, respectively), and all 15 patients had LDH levels below 1.5xULN. None of the 15 patients required transfusions or encountered any thromboembolic events after receiving KP104 treatment. Positive changes were observed in absolute reticulocyte count and bilirubin levels, along with significant enhancements in FACIT-fatigue scores seen in all 3 cohorts, with mean (SD) increases of 13.8 (±7.91), 12.8 (±9.15), and 9.3 (±10.69) for Cohorts 1, 2, and 3, respectively. Dose-dependent reductions in two PD biomarkers, C3b deposition and free C5 levels, further confirmed KP104's dual mechanism of AP and TP inhibition.
KP104 was well tolerated, with no serious adverse events or treatment-emergent adverse events (TEAEs) that led to drug discontinuation or study withdrawal. 10/15 (67%) patients reported at least one mild or moderate TEAE, with no observed dose dependency. The most frequently reported TEAEs included transient injection site induration, headache, and COVID-19 infection, all of which were promptly or duly resolved. A single occurrence of clinical breakthrough hemolysis, observed in the lowest dose cohort and concurrent with an episode of gastroenteritis, was quickly resolved after an extra dose.
Conclusion
The interim results of this phase 2 study demonstrate the safety and efficacy of KP104 in effectively controlling IVH and EVH in complement inhibitor-naïve PNH patients. The findings provide compelling clinical evidence that a bifunctional inhibitor targeting the AP and TP can properly address the current treatment gaps associated with single complement pathway inhibitors, potentially eliminating the need for cumbersome and costly combination therapies (Notaro et al. N Engl J Med 2022;387:160-6).
Disclosures
Wang:Kira Pharmaceuticals: Current Employment. Yue:Kira Pharmaceuticals: Current Employment. Yan:Kira Pharmaceuticals: Current Employment. Ma:Kira Pharmaceuticals: Current Employment. Fu:Kira Pharmaceuticals: Current Employment. He:Kira Pharmaceuticals: Current Employment. Tsui:Kira Pharmaceuticals: Current Employment. Wu:Kira Pharmaceuticals: Current Employment. Lee:Kira Pharmaceuticals: Current Employment. Song:Kira Pharmaceuticals: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal